is co polar or nonpolar Polar nonpolar carbon monoxide polarity jan
Hey there, science enthusiasts! Today, we’re diving into the fascinating world of molecular polarity. Our focus for today is a compound called carbon monoxide (CO) and the question on everyone’s minds: Is CO polar or nonpolar?
Understanding Molecular Polarity
Before we get to the answer, let’s recap what molecular polarity means. In simple terms, it refers to the uneven distribution of electrons within a molecule. If the electrons are shared equally between the atoms, the molecule is considered nonpolar. On the other hand, if there is an uneven distribution of electrons, resulting in partial positive and negative charges, the molecule is polar.
Now, let’s analyze carbon monoxide (CO) to determine its polarity.
The Structure of Carbon Monoxide (CO)
Carbon monoxide is a diatomic molecule consisting of one carbon (C) atom and one oxygen (O) atom, connected by a triple bond. This gives CO a linear molecular shape.
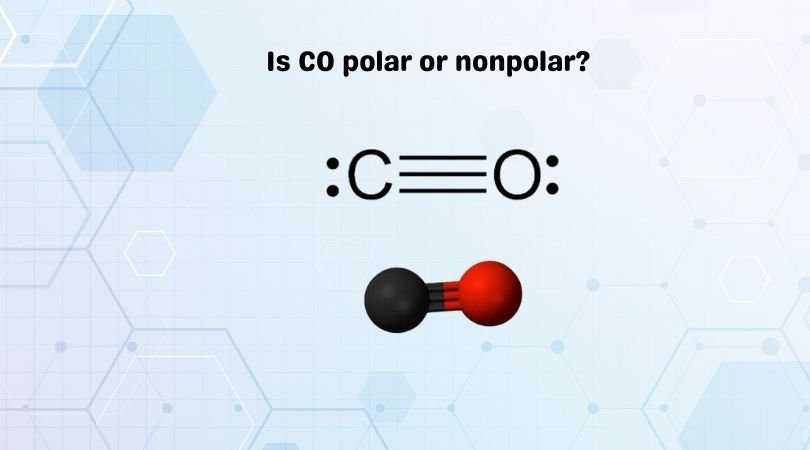 The electronegativity difference between carbon (C) and oxygen (O) is relatively large, with oxygen being more electronegative than carbon. The electronegativity is a measure of an atom’s ability to attract electrons. This electronegativity difference indicates that CO may likely have an uneven distribution of electrons.
The electronegativity difference between carbon (C) and oxygen (O) is relatively large, with oxygen being more electronegative than carbon. The electronegativity is a measure of an atom’s ability to attract electrons. This electronegativity difference indicates that CO may likely have an uneven distribution of electrons.
Determining Polarity
To understand whether CO is polar or nonpolar, we need to consider both the molecular shape and the electronegativity difference.
Due to its linear structure, the dipole moments of the two polar bonds in carbon monoxide should cancel each other out if they have the same magnitude. However, the electronegativity difference between carbon and oxygen causes the molecule to be polar, with oxygen pulling electrons towards itself, creating a partial negative charge on the oxygen atom and a partial positive charge on the carbon atom.
 So, the verdict is in: Carbon monoxide (CO) is indeed a polar molecule.
So, the verdict is in: Carbon monoxide (CO) is indeed a polar molecule.
Implications of CO’s Polarity
Knowing that CO is polar has several implications. For instance, its polarity affects the physical properties of the compound, such as boiling and melting points. Additionally, polarity also influences the solubility of CO in different solvents.
Moreover, the polar nature of carbon monoxide plays a crucial role in its reactivity and how it interacts with other molecules. This can have significant effects on chemical reactions and the role of carbon monoxide in various industrial processes.
So there you have it - the answer to whether carbon monoxide is polar or nonpolar. Understanding molecular polarity helps us gain insights into the behavior and properties of different compounds, making it a fundamental concept in the world of chemistry.
If you found this information intriguing, stay tuned for more fascinating science topics. Until next time, keep exploring and expanding your scientific knowledge!
If you are searching about Is CO Polar or Nonpolar (Carbon monoxide) - YouTube you’ve visit to the right web. We have 5 Pics about Is CO Polar or Nonpolar (Carbon monoxide) - YouTube like Is CO (Carbon Monoxide) polar or nonpolar? : Check Polarity, Is CO Polar or Nonpolar? - Techiescientist and also Is CO Polar or Nonpolar (Carbon monoxide) - YouTube. Here you go:
Is CO Polar Or Nonpolar (Carbon Monoxide) - YouTube
 www.youtube.comIs CO (Carbon Monoxide) Polar Or Nonpolar? : Check Polarity
www.youtube.comIs CO (Carbon Monoxide) Polar Or Nonpolar? : Check Polarity
 geometryofmolecules.compolar nonpolar carbon monoxide polarity jan
geometryofmolecules.compolar nonpolar carbon monoxide polarity jan
MakeTheBrainHappy: Is CO Polar Or Nonpolar?
 www.makethebrainhappy.comnonpolar co2
www.makethebrainhappy.comnonpolar co2
Is CO Polar Or Nonpolar
 www.bengislife.compolar nonpolar non
www.bengislife.compolar nonpolar non
Is CO Polar Or Nonpolar? - Techiescientist
 techiescientist.commonoxide carbono covalent monoxido ikatan kovalen carbone molecule monóxido nonpolar rangkap ionic monoxyde monoksida karbon chemical dative estructural oxide formulacion
techiescientist.commonoxide carbono covalent monoxido ikatan kovalen carbone molecule monóxido nonpolar rangkap ionic monoxyde monoksida karbon chemical dative estructural oxide formulacion
Makethebrainhappy: is co polar or nonpolar?. Polar nonpolar non. Nonpolar co2